Abstract
The BABY HUG clinical trial of hydroxyurea in infants and young children with sickle cell disease (SCD) established the safety and clinical benefit of hydroxyurea therapy in this age group. The study did not establish, however, if escalation to maximum tolerated dose (MTD) could be safely accomplished and resulted in increased benefit in this age group.
We sought to determine the safety and clinical effectiveness of dose-escalation hydroxyurea therapy in children with SCD under the age of two years. We conducted a retrospective chart review of children with HbSS or HbS β0Thalassemia who initiated hydroxyurea therapy before the age of two years between 2011 and 2017 at the Texas Children's Hematology Center. We assessed the clinical and laboratory response to dose-escalation therapy as well as any adverse events associated with hydroxyurea therapy.
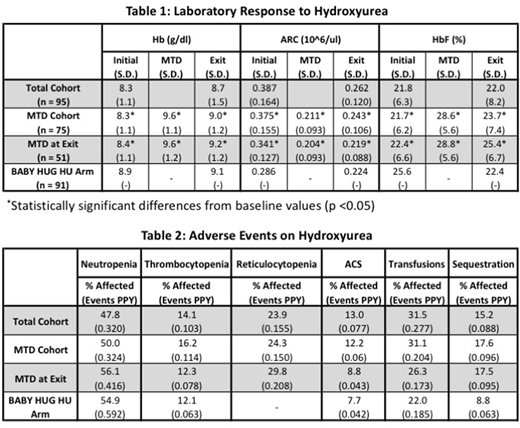
We identified 95 patients ranging from 5 to 24 months in age who were initiated on hydroxyurea therapy during the study period. MTD was achieved in 75 patients (76.5%), of whom 51 were still at MTD at end of monitoring. There were significant increases in hemoglobin concentration (Hb) and hemoglobin F (HbF) levels and decline in absolute reticulocyte count (ARC) on hydroxyurea therapy (Table 1). Additionally, the magnitude of increases in Hb and HbF were greater than those seen in the BABY HUG hydroxyurea treatment group both at the time of MTD and at end of assessment. The proportion of patients experiencing neutropenia or thrombocytopenia as well as the number of events per patient-year (PPY) over 193.9 patient-years were similar to those in the BABY HUG hydroxyurea treatment group (Table 2). The incidence of acute chest syndrome (ACS) and transfusions was similar between the two groups, while episodes of splenic sequestration were more common in patients advanced to MTD.
In summary, hydroxyurea therapy with dose escalation to MTD appears to be safe in infants and young children and results in more significant and sustained increases in hemoglobin concentration and %HbF than were seen with fixed dosing in the BABY HUG study. In the context of this retrospective study, though, hydroxyurea dose escalation did not clearly result in increased clinical benefit relative to fixed-dose treatment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal